


Advanced Analytics
To ensure that our developed drug delivery systems are effective, it is essential to gain a deep molecular understanding of the system itself as well as interactions between the drug and its carrier and between carrier and cells. We strive to achieve this understanding by implementing a range of complementary methods and advanced analysis procedures that characterize our delivery systems during different development steps. We believe necessary adjustments toward carrier optimization can only be made by a comprehensive understanding of the drug delivery system. Therefore, we apply a variety of imaging approaches, methods for the physicochemical analysis and assays for the evaluation of biological effects.
Physico-chemical Analysis
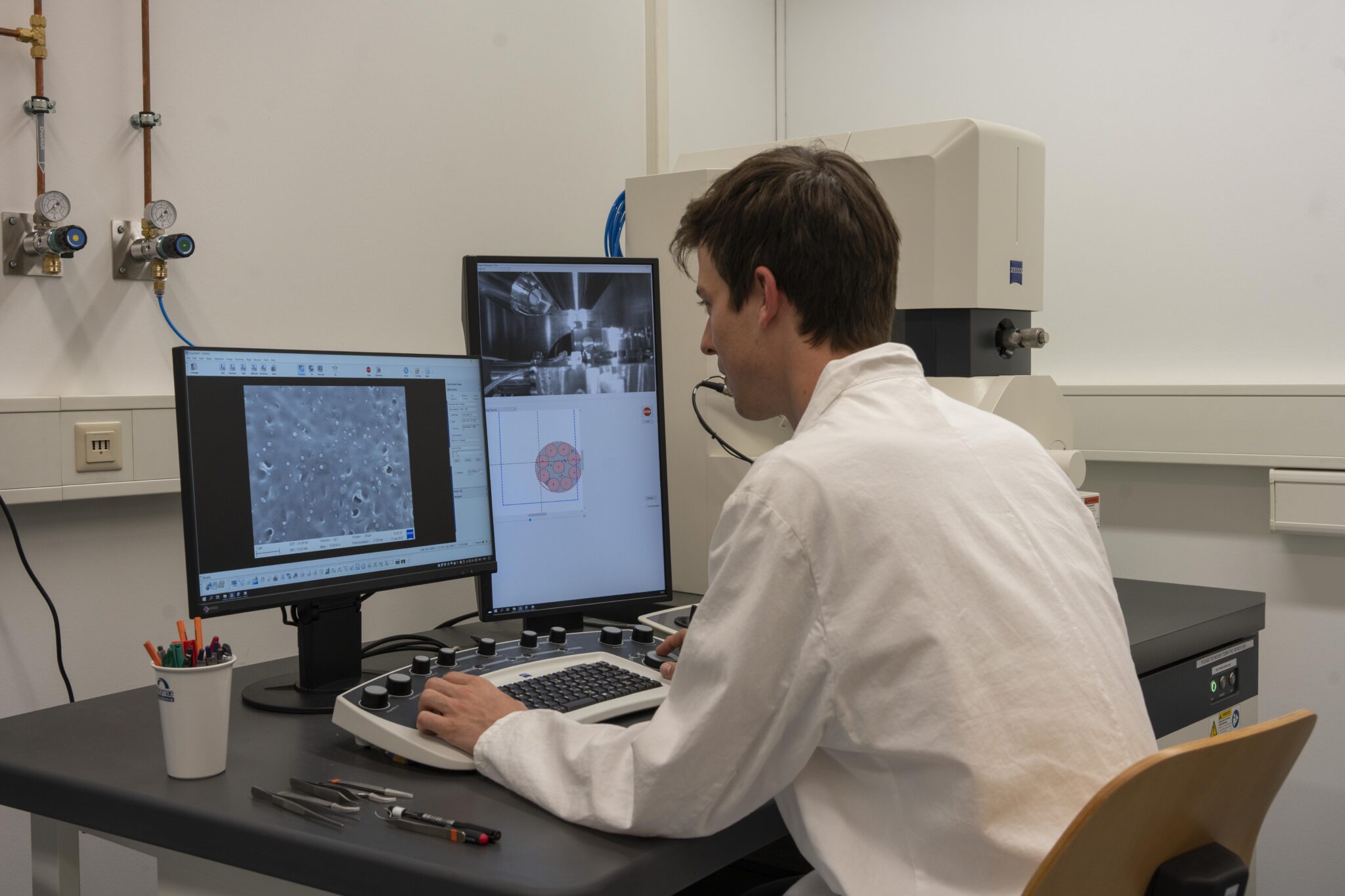
We employ a robust and predictive toolset of analytical techniques to cover a comprehensive investigation of our developed drug delivery systems at different stages during their development. For example, we gain information about the thermal properties of e.g. polymers using differential scanning calorimetry (DSC) , or use attenuated total reflectance infrared spectroscopy (ATR-IR) for compositional analyses to identify functional groups within the sample. Characterization of nanoparticulate formulations is performed using dynamic light scattering (DLS) as well as electrophoretic light scattering (ELS) measurements to determine the size and surface charge of nanoparticles and liposomes. Further, tensile testing and contact angle measurements help us to understand the mechanical properties and the degree of hydrophilicity of nanofibrous systems, respectively. For the characterization of liquid and semi-solid samples, we employ rheology analysis enabling the measurement of viscoelastic properties of whole systems, and nano-indentation, which allows for the investigation of the local stiffness and elastic modulus at small scales. Depending on the encapsulated active ingredients, we use different assays for monitoring drug release from the respective system, including high‑performance liquid chromatography (HPLC) or protein quantification assays. The combination of these techniques together with imaging approaches provides us with a detailed understanding of the physical and chemical properties of our drug delivery systems and their biological performance.
Related Publications
Associated Team Members
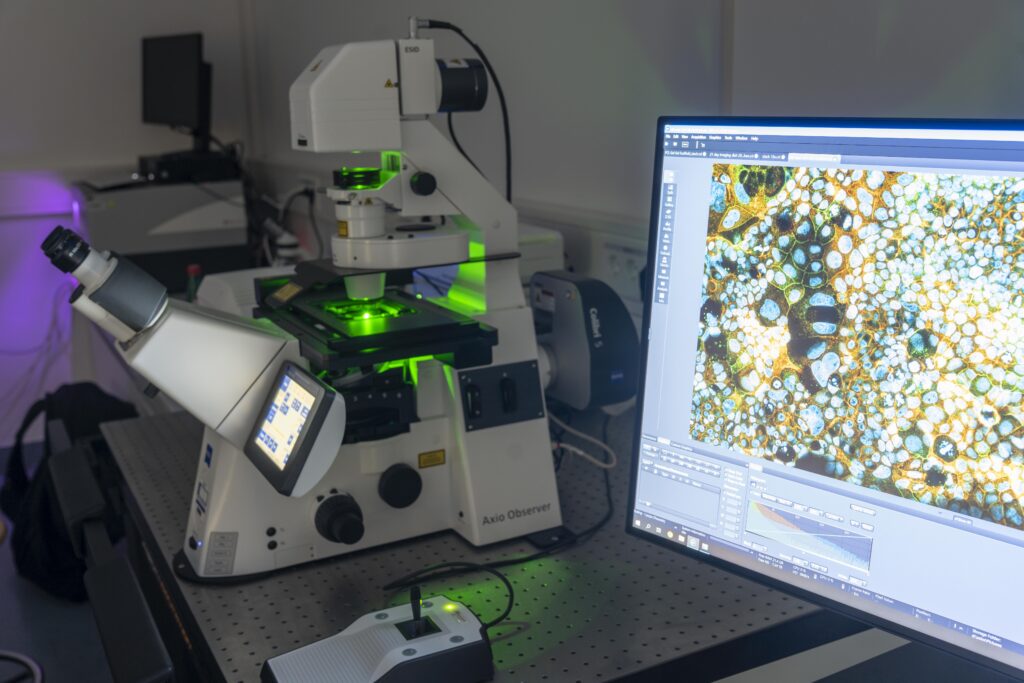
Imaging Approaches
To obtain an in-depth understanding of our drug-loaded carriers as well as their interaction with biological systems, our analytical spectrum includes a range of established and novel imaging techniques. Our in-house imaging laboratory is equipped with a confocal laser-scanning microscope (CLSM) that allows for fluorescence imaging of three-dimensional samples and a scanning electron microscope (SEM) for the high-resolution visualization of nanostructured surfaces. A correlative approach connects both techniques in a seamless workflow for the combined investigation of fluorescently labeled samples and their surface properties. An automated microscope within an incubation chamber further allows live cell imaging of e.g. cell migration and wound healing processes. For the analysis of samples that require non‑destructive or label-free characterization, we employ confocal Raman microscopy (CRM) as a laser-based, high-resolution imaging technique. The acquisition of spatially resolved Raman spectra allows for a discrimination of chemically different components resembling individual “molecular fingerprints” for chemical as well as biological substances. Our applications range from analyses of drug distribution within the carrier and visualization of in situ drug release up to monitoring cell differentiation within our cell- and tissue-based in vitro models and the intracellular fate of our drug carriers.
Related Publications
- Jung N, Namjoshi S, Mohammed Y, Grice JE, Benson HAE, Raney SG, Roberts MS, Windbergs M. Application of Confocal Raman Microscopy for the Characterization of Topical Semisolid Formulations and their Penetration into Human Skin Ex Vivo. Pharm Res. 2022; 39(5):935-948. doi: 10.1007/s11095-022-03245-7
- Jung N, Moreth T, Stelzer EHK, Pampaloni F, Windbergs M. Non-invasive analysis of pancreas organoids in synthetic hydrogels defines material-cell interactions and luminal composition. Biomater Sci. 2021; 9(16):5415-5426. doi: 10.1039/d1bm00597a
- Fahier J, Vukosavljevic B, De Kinder L, Florin H, Goossens JF, Windbergs M, Siepmann F, Siepmann J, Muschert S. Towards a Better Understanding of Verapamil Release from Kollicoat SR:IR Coated Pellets Using Non-Invasive Analytical Tools. Pharmaceutics. 2021; 13(10):1723. doi: 10.3390/pharmaceutics13101723
- Vukosavljevic B, Murgia X, Schwarzkopf K, Schaefer UF, Lehr CM, Windbergs M. Tracing molecular and structural changes upon mucolysis with N-acetyl cysteine in human airway mucus. Int J Pharm. 2017; 533(2):373-376. doi: 10.1016/j.ijpharm.2017.07.022
Associated Team Members
Biological Analysis
With respect to a holistic approach of our drug delivery systems evaluation, we implement a range of biological assays to evaluate the interaction between drug carriers and cells or tissues. These assays are used to determine the cytotoxicity, immunogenic potential, or other detrimental effects of the released drug molecule or the carrier system itself. We further employ fluorescence-based techniques to quantify the uptake of nanocarriers into single cells and their transport across endothelial or epithelial barriers. Additionally, we investigate the effectiveness of the drug carrier in delivering its therapeutic payload to the cells or tissues by monitoring immune responses (e. g., ELISA), the effect on protein expression, or wound healing abilities of drug molecules in real-time. Global alterations of whole tissues are further analysed using histology approaches, from standard paraffin embedding to the implementation of resins for more delicate samples. Overall, the implementation of biological assays is an important tool for evaluating the safety and efficacy of drug carriers in the development of new therapeutics.

Related Publications
- Brettner FEB, Schreiner J, Vogel-Kindgen S, Windbergs M. Engineered Self-Assembly of Amphiphilic Cyclodextrin Conjugates for Drug Encapsulation. ACS Biomater Sci Eng. 2022. doi: 10.1021/acsbiomaterials.2c01023
- Millies B, von Hammerstein F, Gellert A, Hammerschmidt S, Barthels F, Göppel U, Immerheiser M, Elgner F, Jung N, Basic M, Kersten C, Kiefer W, Bodem J, Hildt E, Windbergs M, Hellmich UA, Schirmeister T. Proline-Based Allosteric Inhibitors of Zika and Dengue Virus NS2B/NS3 Proteases. J Med Chem. 2019;62(24):11359-11382. doi: 10.1021/acs.jmedchem.9b01697
- Planz V, Wang J, Windbergs M. Establishment of a cell-based wound healing assay for bio-relevant testing of wound therapeutics. J Pharmacol Toxicol Methods. 2018; 89:19-25. doi: 10.1016/j.vascn.2017.10.003
- Planz V, Lehr CM, Windbergs M. In vitro models for evaluating safety and efficacy of novel technologies for skin drug delivery. J Control Release. 2016; 242:89-104. doi: 10.1016/j.jconrel.2016.09.002










